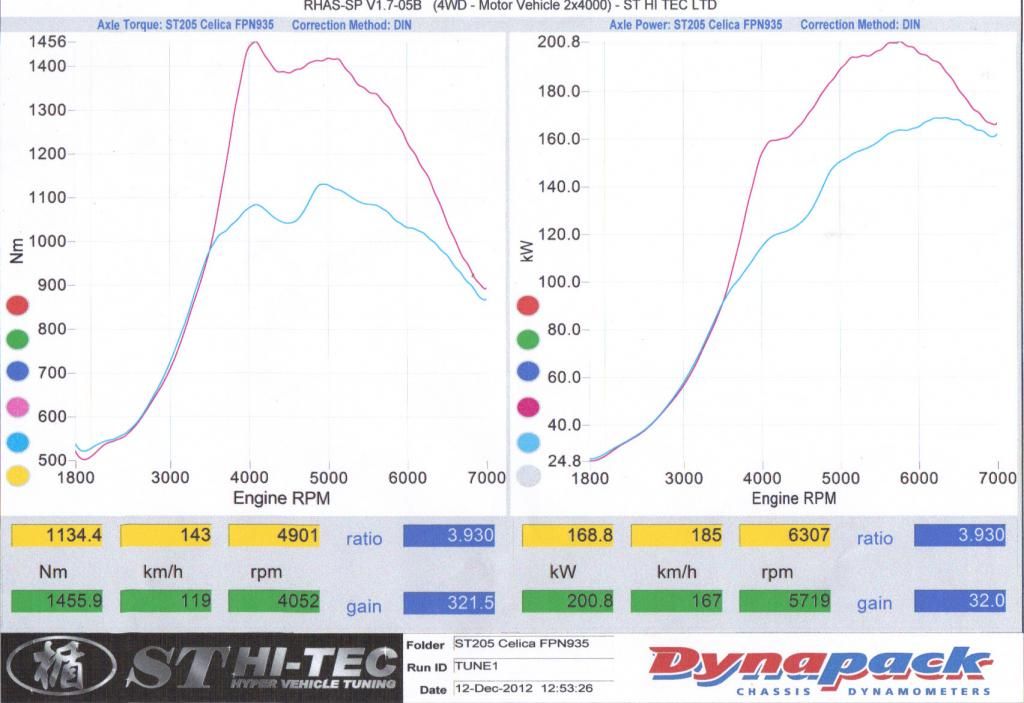
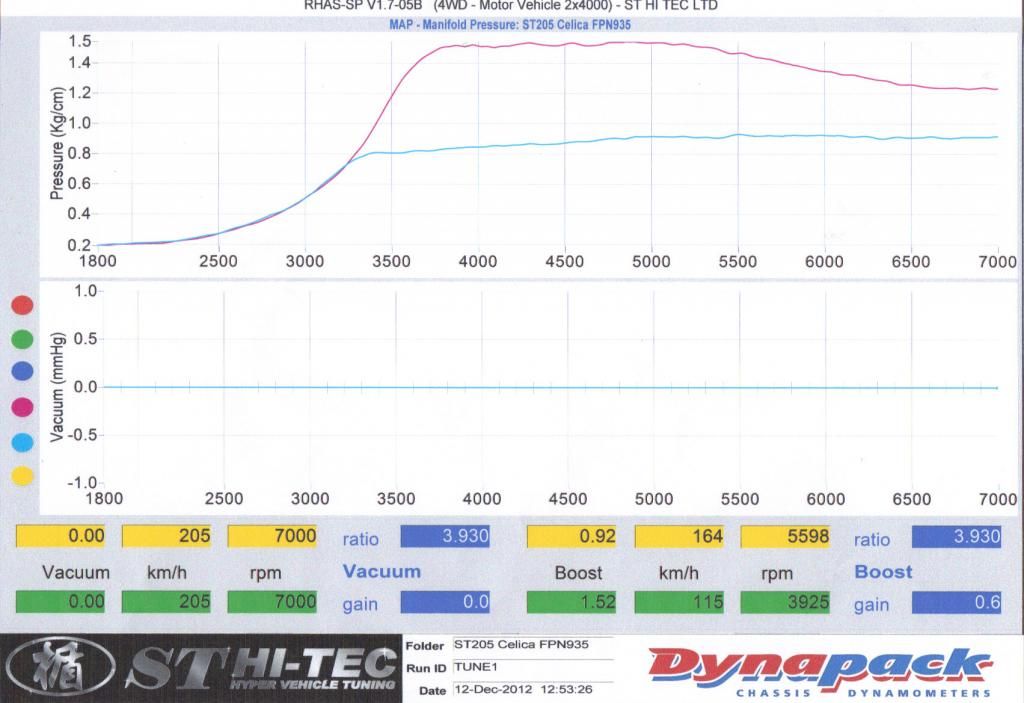
Lith wrote:RedMist wrote:Ideal gas law. PV = nRT
Given temperature (T), volume(V) and gas constant (R) are constants. Any increase in pressure is a directly proportional increase in moles. Double pressure, double moles.
I am very familiar with all of the above, but thanks for re-re-iterating. So you actually know why your car made proportionally more power than "P" and just sharing the brain teaser, or you don't know why but aren't willing to explore the option that if you are getting results you aren't expecting then it is inevitable there are parts of the puzzle you are missing?
No, I simply have no idea. It defies my logic, hence my logic is wrong. It's an interesting quandary, one that I'm happy exists, but still haunts me. I'm certainly willing to explore options, however your answer is also not logical. I'm not discounting it on any other basis than the only options for your answer to be correct is for either the gas constant to change or for temperature to fall in the turbocharged installation.
Pressure is set (at two values), volume (of the stroke) is set, lets assume the gas constant is set, T, n are the only values you can change and they are not independent. Change one, and you must inversely effect the another.
I believe that there is an external force in act. That being fuel. Either I'm getting significant amounts of O2 from the fuel (E30) or the fuel is supplying a more constant torque force to the piston head. Both of which are true to a minor extent however given stoichiometric, and Octane values for E30. However I simply can't see that is true for the amount of power I'm getting over and above less than a bar of boost.